The medical writing profession is undergoing a significant transformation in 2025. With the global market expanding to $5.18 billion from $4.66 billion in 2024—and projected to reach $12.12 billion by 2033—the integration of automation technology into healthcare documentation represents the most significant industry shift in decades. This comprehensive guide explores medical writing trends, regulatory requirements, career opportunities, and strategic insights for healthcare professionals navigating this dynamic landscape.
Understanding Medical Writing Trends 2025: Market Expansion and Professional Opportunities
The medical writing industry continues its impressive growth trajectory. With specialized focus on regulatory medical writing, clinical study report automation, and healthcare documentation software, organizations are investing heavily in modernizing how health information is created and disseminated. The market segment dedicated to document generation technology has expanded from $878 million in 2024 to $1.15 billion in 2025, marking an 11.2% annual growth rate.
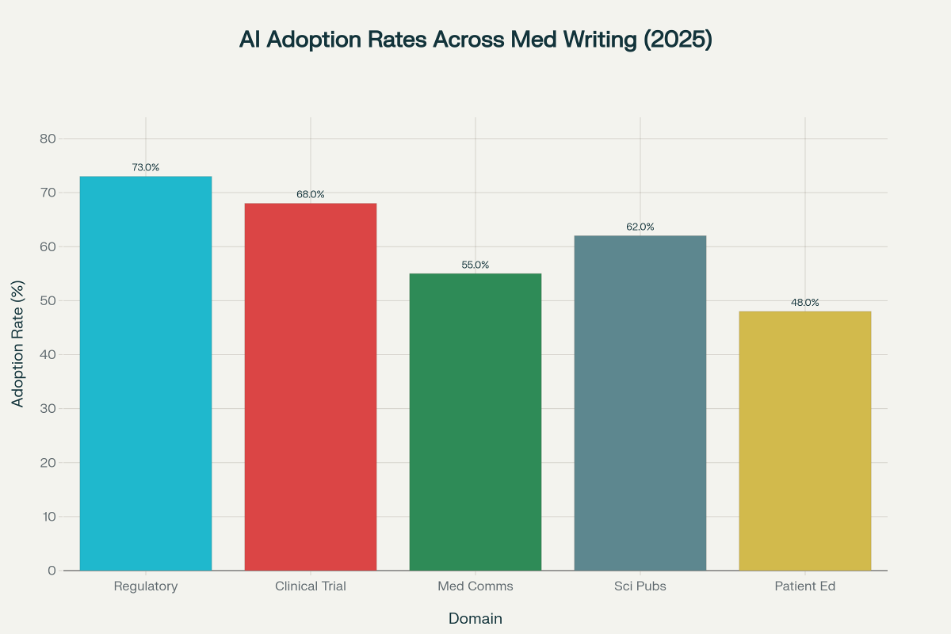
The adoption of automation technology for healthcare documentation has progressed beyond pilot programs to mainstream deployment. 73% of major pharmaceutical organizations now actively employ advanced software tools for regulatory compliance and clinical study report automation, with measurable time savings ranging from 30-40% compared to traditional manual methods. Organizations report cost reductions of 30-40% per document while simultaneously improving consistency and accuracy.
Clinical Study Report Automation: How Technology Transforms Medical Documentation
Clinical study report automation represents one of the most practical applications of modern technology in medical writing. Rather than spending weeks manually compiling data and drafting reports, healthcare professionals now use document generation technology that can synthesize information from multiple sources and organize it according to regulatory medical writing standards. This efficiency gain means writers can spend less time on mechanical documentation and more time on meaningful analysis and interpretation.
The business case is straightforward: document generation tools compress timelines from weeks to days, while built-in compliance checking mechanisms reduce regulatory delays. Research demonstrates that these systems achieve strong accuracy ratings, though human oversight remains essential to verify all facts against primary sources. Professional guidelines increasingly emphasize that automation technology functions as an augmentation tool requiring rigorous human oversight, not a replacement for medical writing expertise.
Plain Language Summaries: Meeting Regulatory Requirements and Reader Expectations
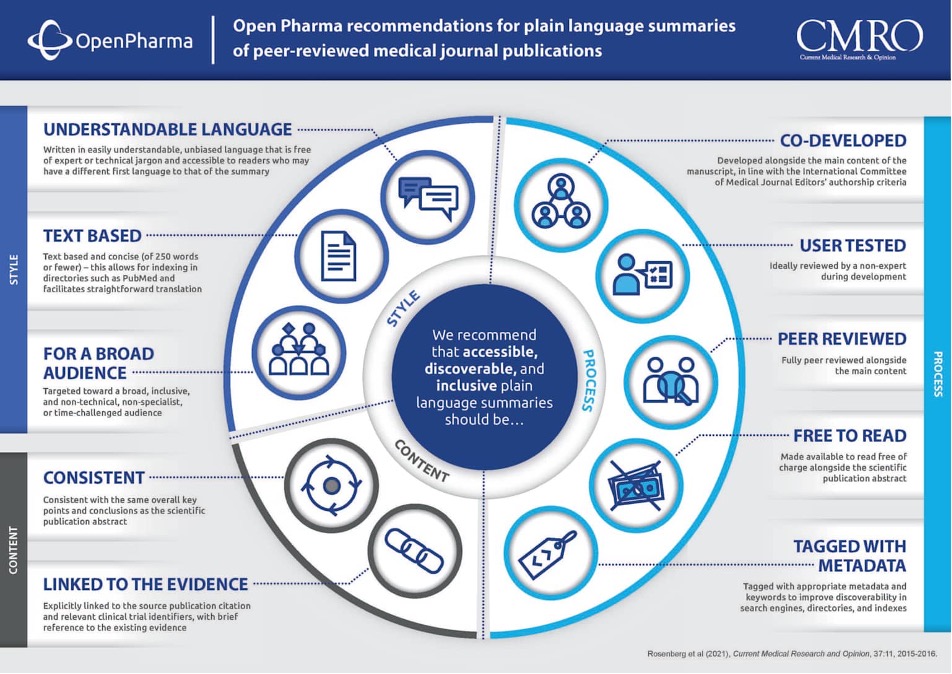
Plain language summaries have transitioned from optional communication to a regulatory requirement under FDA clinical trial requirements and European Medicines Agency guidelines. This regulatory mandate directly impacts healthcare content writing workload: 48% of healthcare organizations have implemented automation tools for plain language summary generation, though human editorial review remains essential for accuracy verification.
Understanding reader preferences is crucial for healthcare content writing success. Research reveals that more than 50% of patients prefer graphical summaries over text alone, while text-based medical documents rate highest at medium complexity (reading age 14-17 years). These findings challenge conventional assumptions and suggest patients value scientific authenticity balanced with accessibility, a key principle in modern healthcare content writing.
Best-practice healthcare content writing incorporates collaborative development with patient representatives, user testing, and rigorous human review before publication.

Real-World Evidence Healthcare: Expanding Evidence Standards in Modern Documentation
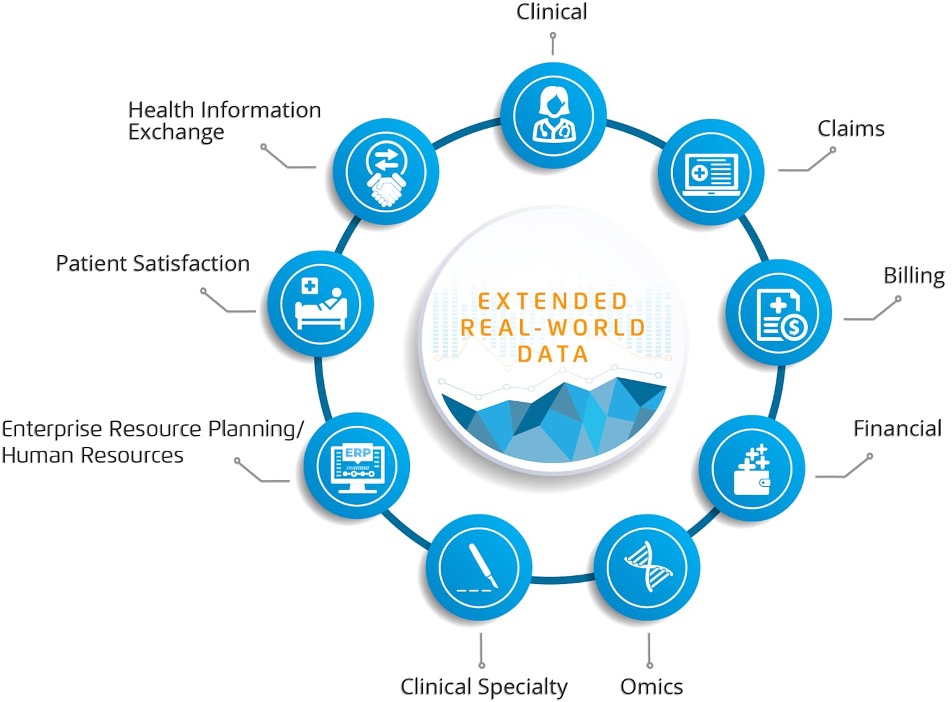
Real-world evidence in healthcare represents a fundamental shift in how medical professionals document clinical outcomes. 85% of pharmaceutical regulatory submissions now include real-world evidence components, derived from electronic health records, insurance claims databases, patient registries, and wearable device data. This expansion reflects recognition that routine clinical practice data provides valuable insights beyond controlled trial environments.
The FDA’s recognition of real-world evidence healthcare through formal frameworks has legitimized this approach, enabling therapeutic decisions based on pragmatic data from routine clinical practice. The FDA’s 2019 label expansion for IBRANCE® (palbociclib) relied substantially on real-world evidence from electronic health records, demonstrating direct precedent for how clinical documentation using real-world data drives therapeutic decisions. This trend accelerates market demand for healthcare professionals skilled in data synthesis and rigorous methodology documentation.
Digital Therapeutics Documentation: Specialized Medical Writing for App-Based Therapies
Digital therapeutics documentation represents an emerging specialization within medical writing careers. 51% of healthcare organizations implementing specialized digital therapeutics documentation protocols recognize that app-based therapies require unique documentation approaches. Unlike traditional pharmaceutical documentation, digital therapeutics products continuously iterate, requiring healthcare professionals to address software version control, security protocols, usability testing, and clinical evidence documentation simultaneously.
The regulatory landscape for digital health writing remains dynamic. While expedited review pathways exist, reimbursement pathways remain uncertain, forcing medical writing professionals to articulate clinical value propositions addressing multiple stakeholder concerns.
Medical Writing Career Path: Job Market Growth and Professional Development
The medical writing job market continues to expand, with healthcare organizations actively recruiting qualified professionals. Medical writing jobs continue growing at 11.2% annually, with particular demand for specialists in emerging therapeutic areas. Professionals seeking a medical writing career path should consider specialized expertise in regulatory compliance, healthcare, pharmacovigilance report writing, or digital therapeutics documentation, which command premium compensation.
Professional development remains essential for career advancement. Medical writing certification programs, specialized courses, and continuing education opportunities help professionals develop expertise in specific domains. The most successful medical writing careers combine broad knowledge with specialized expertise, whether in regulatory medical writing, healthcare communications strategy, or emerging therapeutic areas.
Medical Writer Salary and Compensation Trends
Compensation in the medical writing profession reflects growing demand and specialized expertise requirements. The medical writer salary landscape shows significant variation based on specialization, experience, and geographic location. Professionals with demonstrated expertise in emerging therapeutic areas, regulatory compliance, healthcare, or healthcare documentation software earn 25-35% higher compensation than generalist professionals.
Remote medical writing opportunities have expanded compensation possibilities by eliminating geographic limitations.
Medical Writing Certification: Professional Credentialing and Educational Pathways
Professional credentialing through medical writing certification programs has become increasingly important for career advancement. Medical writing certification demonstrates competency in regulatory standards, healthcare documentation practices, and industry best practices. Many employers prioritize candidates with formal medical writing certification when hiring for regulatory medical writing and pharmaceutical documentation roles.
Regulatory Medical Writing: Navigating Compliance and Standards
Regulatory medical writing encompasses documentation required for drug approvals, device submissions, and clinical trial compliance. Regulatory compliance in healthcare represents one of the most critical functions in the pharmaceutical industry. Professionals specializing in regulatory medical writing understand FDA clinical trial requirements, ICH guidelines, and other international regulatory standards.
Healthcare regulatory requirements now address digital therapeutics, precision medicine products, and complex biologics, therapeutic areas requiring specialized documentation expertise.
Pharmacovigilance Report Writing and Medical Communications Strategy
Pharmacovigilance report writing addresses the critical function of monitoring drug safety and adverse events. Professionals specializing in pharmacovigilance report writing synthesize safety data, identify potential signals, and prepare documentation for regulatory submission.
Medical communications strategy encompasses how healthcare organizations communicate scientific findings to diverse audiences, healthcare providers, patients, policymakers, and the media. An effective medical communications strategy balances scientific accuracy with accessibility.
Healthcare Documentation Software and Best Practices
Healthcare documentation software solutions have evolved significantly, offering document generation, version control, compliance checking, and collaborative writing capabilities. Modern healthcare documentation software streamlines workflows and improves productivity for medical writing teams.
Medical writing best practices emphasize accuracy, clarity, consistency, and compliance throughout the documentation lifecycle. Rigorous fact-checking, clear communication, consistent terminology, regular revision cycles, and compliance with regulatory standards form the foundation of professional medical writing.
Healthcare Regulatory Requirements and Emerging Standards
Healthcare regulatory requirements continue evolving as regulatory agencies adapt guidelines to accommodate novel therapies and emerging evidence. Recent updates to FDA clinical trial requirements address digital health technologies, real-world evidence integration, and expedited pathways for breakthrough therapies.
Telemedicine documentation standards represent an emerging area of focus for healthcare organizations implementing telehealth services. Professional organizations are developing specific telemedicine documentation standards to ensure consistency, compliance, and quality in virtual healthcare settings.
Market Segmentation and Industry Growth
The medical writing industry segments into distinct healthcare specializations with varying growth trajectories:
| Medical Writing Specialization | 2025 Market Share | Primary Market Driver | Professional Adoption |
| Clinical Writing Services | 35.6% | Increased global clinical trial volume | 68% |
| Regulatory Writing Expertise | 28.2% | Complex biologics and pharmaceutical submissions | 73% |
| Medical Communications Strategy | 21.5% | Healthcare scientific engagement programs | 55% |
| Publications and Manuscript Services | 14.7% | Open science and healthcare research initiatives | 70% |
Medical writing industry growth continues to accelerate, driven by increasing clinical trial volumes, regulatory complexity, and healthcare communication demands. The global medical writing market is expected to expand to $12.12 billion by 2033. The Asia Pacific region leads the regional medical writing market growth.
Natural Language Processing, Healthcare, and Professional Success
Natural language processing healthcare applications help automate portions of documentation tasks, extract information from unstructured data, and improve documentation accuracy. Healthcare professionals working with these systems emphasize the importance of human oversight and quality assurance.
Key Strategic Imperatives:
- Develop Specialized Expertise – Build expertise in regulatory compliance healthcare, digital therapeutics documentation, or emerging therapeutic domains.
- Invest in Continuous Learning – Pursue medical writing certification programs and stay current with evolving requirements.
- Master Healthcare Documentation Software – Develop proficiency with industry-standard platforms.
- Build Patient-Centered Communication Skills – Strengthen ability to create clear, accessible healthcare content.
- Understand Real-World Evidence Integration – Develop expertise in healthcare data analysis and methodology documentation.
Conclusion: Future Opportunities in Medical Writing
Medical writing in 2025 stands at the intersection of technological transformation, regulatory evolution, and market expansion. Healthcare professionals thriving in this environment combine technical proficiency with unwavering commitment to accuracy, clarity, and ethical accountability.
The medical writing job market continues to expand, with sustained demand for qualified professionals across all specializations. Medical writing careers offer competitive compensation, professional growth opportunities, and the satisfaction of contributing meaningfully to healthcare advancement. Success requires continuous learning, ethical commitment to accuracy, and strategic focus on advancing patient health and enabling informed healthcare decision-making.
| Medical Writing Specialization | Professional Tool Adoption | Efficiency Improvements | Primary Applications |
| Regulatory Writing | 73% | 30-40% | Clinical study report generation, regulatory compliance |
| Manuscript Preparation | 70% | 30-40% | Abstract generation, scientific document formatting |
| Pharmacovigilance Writing | 65% | 35-45% | Safety event detection, healthcare safety reporting |
| Clinical Trial Documentation | 68% | 35-45% | Clinical data synthesis, statistical reporting |
| Medical Communications | 55% | 25-35% | Healthcare content personalization, multi-channel delivery |
| Patient Education | 48% | 20-30% | Plain language healthcare conversion |
| Medical Device Documentation | 51% | 30-40% | Technical healthcare documentation |
| Healthcare Professional Focus Area | Market Impact (1-10) | Implementation Timeline | Key Professional Challenge |
| Medical Writing Trends 2025 | 10 | Currently active (2024-2025) | Staying updated with rapid changes |
| Plain Language Summaries | 9 | Current regulatory requirement (2024-2025) | Balancing simplicity with scientific accuracy |
| Real-World Evidence Healthcare | 8 | Ongoing expansion (2025-2027) | Data quality assurance and standardization |
| Digital Therapeutics Documentation | 8 | Accelerating (2025-2026) | Emerging regulatory pathway clarity |
| Patient-Centric Healthcare Communication | 9 | Current priority (2024-2026) | Diverse audience needs and cultural sensitivity |
| Healthcare Regulatory Requirements | 9 | Immediate implementation (2024-2025) | Compliance assurance and documentation accuracy |